Scientists reveal new mechanisms of T cell receptor signaling, which may lead to
Recently, Professor Xu Tao and his team from the Sun Yat-sen University School of Medicine have, for the first time, demonstrated that TCR (T-cell receptor) signals can participate in determining the direction of T-cell differentiation, providing new insights into the differentiation of TH17 cells and also offering new drug targets for the treatment of TH17-related autoimmune diseases.
The research team is very hopeful that drug development targeting the TCR-Lck/Fyn-STAT3 axis can enter clinical trials. They also look forward to the compounds they have developed entering clinical trials and ultimately being used for the treatment of patients with TH17 cell-related autoimmune diseases.
"This study is also the first step in realizing my long-standing dream, and the relevant international patents are already in the application process," said Xu Tao.
Subsequently, we will seek all possible resources, such as various research funds and venture capital, to bring this achievement to the clinic.The Journey of RORγt Inhibitors: A Long and Winding Road
TH17 cells are a subset of cells that differentiate from antigen-stimulated CD4+ naive T cells and are capable of secreting interleukins such as IL-17, IL-17F, and IL-22.
In autoimmune diseases such as multiple sclerosis, rheumatoid arthritis, psoriasis, ankylosing spondylitis, inflammatory bowel disease, asthma, and systemic lupus erythematosus, TH17 cells play a very important pathogenic role.
Many of these diseases are also referred to as "incurable" cancers, such as rheumatoid arthritis, which torments patients over the long term.
To achieve the differentiation of TH17 cells, the involvement of cytokines such as TGFβ, IL-1β, IL-6, and IL-23 is required.In the text, the cytokines IL-6 and IL-23 are both transcribed through the JAK-STAT3 pathway to regulate the master transcription factor RORγt of TH17 cells, as well as control other characteristic genes, thereby mediating the differentiation of TH17 cells.
Currently, cytokine-blocking antibodies that can induce the differentiation of TH17 cells have been approved for marketing. Specifically:
Blocking antibody drugs targeting IL6/IL6R, such as Tocilizumab, Sarilumab, Siltuximab, and Situximab, and blocking antibody drugs targeting IL-23, such as Risankizumab, as well as blocking antibody drugs targeting the cytokine IL-17 secreted by TH17 cells, such as Secukinumab, have all been approved by the U.S. Food and Drug Administration (FDA, Food and Drug Administration).
However, these antibody drugs generally have a large molecular weight, making it difficult to reach special areas such as the central nervous system, and they are relatively expensive.
The small molecule drugs currently approved in the clinic are all inhibitors of JAK kinase. These drugs not only inhibit the downstream signaling pathways of IL-6 and IL-23 but also inhibit the downstream signaling of more than 50 cytokines including IL-4, IL-10, IL-12, and IL-15.And these cytokines are extensively involved in various life processes such as development, hematopoiesis, and immunity through the JAK kinase-STAT pathway.
Due to the relatively large side effects of these small molecule drugs, they have received a black box warning from the FDA.
Clinical studies have shown that patients treated with JAK inhibitors are prone to the recurrence of other viral diseases they carry, such as the recurrence of hepatitis viruses and herpes viruses.
Despite more than a decade of research on TH17, there are only a few drug targets for TH17 small molecules, such as RORγt. Moreover, no RORγt inhibitor has successfully entered phase 3 clinical trials.
Therefore, in the development of small molecule drugs for autoimmune diseases, there is an urgent need to discover safer and more effective drug targets and candidate drugs.For the differentiation of various CD4+ T cell subsets, they share a common characteristic: that is, they all require antigen stimulation signals, co-stimulatory signals, and cytokine signals.
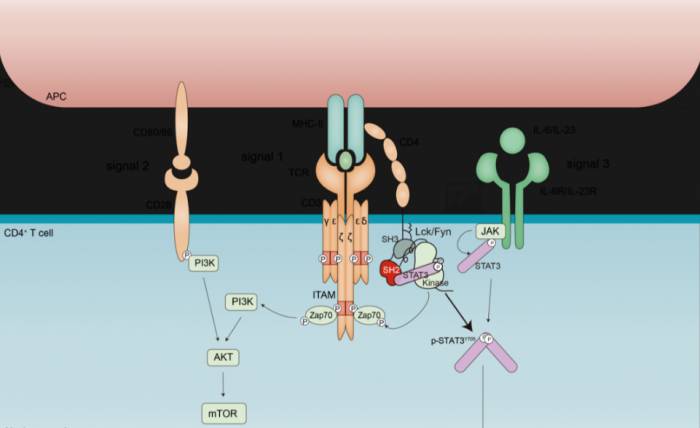
At present, classical immunology textbooks at home and abroad all believe that TCR signals and co-stimulatory signals promote the activation and proliferation of T cells, and this is also a prerequisite for the differentiation of all T cell subsets.
However, TCR signals cannot determine the direction of T cell differentiation. On the contrary, for which T cell subset CD4+ T cells can be differentiated into, cytokine signals are a decisive factor.
Because of this, it is commonly believed that in the differentiation process of TH17 cells, the cytokines IL-6 and IL-23 can phosphorylate STAT3 through JAK kinase.
After phosphorylation, STAT3 enters the nucleus of the cell, which can regulate a series of core transcription factors and characteristic genes related to TH17, thereby mediating the differentiation of TH17 cells."Could it be that our assumptions are wrong?"
In this study, Xu Tao and others found that:
Stimulation by a single cytokine can only induce a moderate degree of STAT3 phosphorylation.
Stimulation by a single TCR can directly induce STAT3 phosphorylation through Lck/Fyn kinases and, through synergy with cytokines, can induce the maximum degree of STAT3 phosphorylation, thereby mediating the differentiation of TH17 cells.
When using selective small molecule inhibitors of Lck/Fyn (Srci1), or disrupting the interaction between Lck/Fyn and STAT3 through pathogenic STAT3 mutations, the following two effects can be achieved:Firstly, it can selectively inhibit STAT3 activation induced by TCR stimulation and the differentiation of TH17 cells.
Secondly, it can transdifferentiate into FOXP3+Treg cells, thereby significantly improving the severity of autoimmune encephalomyelitis mediated by TH17 cells.
Through this, the team has updated the understanding that only pro-inflammatory cytokines can activate STAT3 during the differentiation process of TH17 cells.
It has revealed this new rule: the TCR-Lck/Fyn axis can directly phosphorylate STAT3, and can synergistically induce STAT3 phosphorylation with cytokine signals, thereby promoting the differentiation of TH17 cells.
It should be pointed out that during the differentiation process of TH17 cells, the role of STAT3 cannot be replaced by any other cytokines or genes.The cytokines and transcription factors that play an important role in the differentiation process of TH17 cells can be partially replaced by other cytokines or genes.
For example, the function of RORγt can be partially replaced by RORα, and the role of IL-6 can be partially replaced by IL-21.
It can be seen that the deficiency of STAT3 in T cells completely blocks the differentiation of TH17 cells. Other cytokines and core transcription factors related to TH17, such as the deficiency of IL-6 and RORγt, only partially inhibit the differentiation of TH17 cells.
Therefore, the importance of STAT3 for the differentiation of TH17 cells is far greater than that of any other gene, that is to say, STAT3 is equivalent to the "master switch" for the differentiation of TH17 cells.
On the other hand, systemic deficiency of STAT3 can cause embryonic development to be lethal. Small molecule inhibitors that completely block STAT3 phosphorylation have been proven to have significant side effects.This study demonstrates that the phosphorylation of STAT3 induced by TCR stimulation plays a crucial role in the differentiation process of TH17 cells, a finding that offers a completely new therapeutic approach for the treatment of TH17 cell-related immune diseases.
At the same time, highly selective inhibitors of Lck/Fyn, such as Srci1, can selectively inhibit the phosphorylation of STAT3 induced by TCR stimulation without affecting the phosphorylation of STAT3 induced by cytokines.
For TH17-related autoimmune diseases, this inhibitor is undoubtedly a very promising candidate drug.
As previously mentioned, textbooks both domestically and internationally clearly state that TCR signals and co-stimulatory signals promote the activation and proliferation of T cells, while the cytokine-JAK-STAT signaling pathway determines the differentiation of cells into which T cell subset.
For this reason, the phosphorylation of STAT3 in T cells is considered to be induced by cytokines. When Xu Tao's team first experimentally proved that TCR stimulation alone could induce the phosphorylation of STAT3, they could hardly believe their eyes.For this purpose, Xu Tao and the doctoral students conducted a variety of experiments of more than a dozen different types after repeated discussions.
Similarly, after they used the AlphaFold Multimer tool to discover the interaction region between Lck/Fyn and STAT3, they synthesized peptide fragments containing this region.
Like many people, they took it for granted that adding these peptides would block the interaction between Lck/Fyn and STAT3, thereby inhibiting the phosphorylation of STAT3 and the differentiation of TH17 cells.
However, the results were once again contrary to expectations. Adding these peptides actually promoted the phosphorylation of STAT3 and the differentiation of TH17 cells.
"Could our hypothesis be wrong? We were once again caught in a state of perplexity. For this reason, we read the early literature on how Lck/Fyn regulates the phosphorylation of substrates, and finally discovered the mystery," said Xu Tao.In summary, the process of scientific research requires continuous critical thinking, which means being able to challenge tradition as well as learning from predecessors.
The related paper was eventually published in the Journal of Experimental Medicine (IF 15.3) with the title "TCR signaling induces STAT3 phosphorylation to promote TH17 cell differentiation."
Zhongshan School of Medicine at Sun Yat-sen University doctoral student Qin Zhen and postdoctoral fellow Wang Ruining are the co-first authors, and Professor Xu Tao from Zhongshan School of Medicine at Sun Yat-sen University serves as the corresponding author.
For a long time, engaging in original scientific research and translating these achievements into clinical practice has been a dream that has been lingering in Xu Tao's heart.
In fact, this is something that is easier said than done. It requires a deep understanding of basic biology and innovative discoveries, as well as repeated verification of targets.It is also necessary to screen small molecule compounds or antibodies according to the characteristics of the target, and it is even more important that these small molecules or antibodies not only have clear therapeutic effects but also ensure that these preparations have no obvious toxic side effects on the human body.
Several years ago, after completing his postdoctoral training, Xu Tao did not immediately establish a laboratory, but chose to join the pharmaceutical giant Amgen to learn the process of drug development from scratch, drawing on the experience of large companies in transforming results.
He said: "It is not an exaggeration to say that the time I spent working at Amgen greatly broadened my horizons and provided a lot of help in the choice of topics after establishing the laboratory." In the future, he will also continue to work hard with his team to continue the efforts to transform the results of this study.
POST A COMMENT